Sep 24 2019
An international team of scientists has observed, in real time, the way in which football molecules formed of carbon atoms explode in an X-ray laser beam.
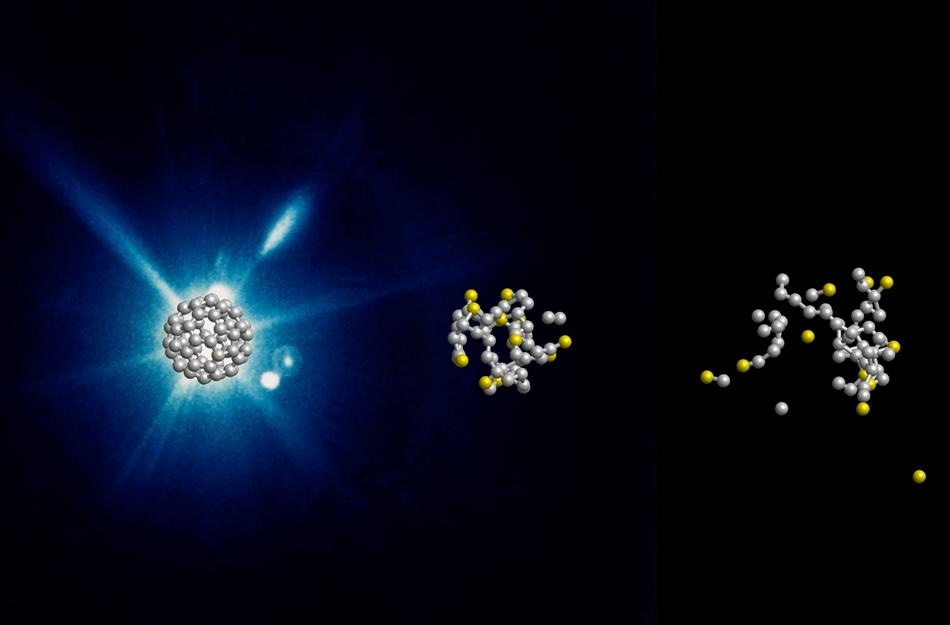 Computer-simulated evolution of a C60 molecule at 0, 60, and 240 fs after the X-ray flash. (Image credit: DESY, Zoltan Jurek)
Computer-simulated evolution of a C60 molecule at 0, 60, and 240 fs after the X-ray flash. (Image credit: DESY, Zoltan Jurek)
The research demonstrates the temporal path of the explosion process, which occurs within a trillionth of a second. This process is crucial for analyzing sensitive proteins and other biomolecules, which are often studied using bright X-ray laser flashes.
In contrast to what is expected, the football molecules burst more gradually and differently, as reported in the Nature Physics journal by the team of Nora Berrah from the University of Connecticut and Robin Santra from DESY. This observation enables a more in-depth protein analysis using X-ray free-electron lasers (XFEL).
The team had performed experiments using buckminster fullerenes, or buckyballs. These spherical molecules are composed of 60 carbon atoms distributed in alternating hexagons and pentagons, similar to the leather coat of a football.
Buckyballs are well suited as a simple model system for biomolecules. Since they consist of only one type of atom and have a symmetrical structure, they can be well represented in theory and experiment. This is a first step before the investigation of molecules from different types of atoms.
Robin Santra, Lead Scientist, Center for Free-Electron Laser Science (CFEL), DESY
Santra is also a physics professor at the Universität Hamburg.
The researchers used the X-ray laser Linac Coherent Light Source (LCLS) at the SLAC National Accelerator Laboratory in California to shoot short X-ray flashes of around 20 fs (or one quadrillionth of 1 second) duration at individual football molecules. Then, they observed the effect in real time using a temporal resolution of about 10 fs.
The data reveal that the X-ray flash removes electrons out of nearly one in five of the 60 carbon atoms. “After that, nothing happens for some time. Only after a few dozen femtoseconds do carbon atoms gradually detach from the molecule,” noted Santra.
What follows then is not an actual explosion. Instead, the buckyballs dissolve comparatively slowly. Carbon atoms gradually evaporate—with many more neutral ones than electrically charged ones, which was surprising.
Robin Santra, Lead Scientist, Center for Free-Electron Laser Science (CFEL), DESY
The disintegration of the buckyballs on this time scale does not occur in an explosive manner but very slowly; therefore, the team discussed the evaporation of the atoms. It would be possible to meaningfully interpret the experimental data only by theoretical modeling of the process.
“Typically, about 25 neutral and only 15 electrically charged carbon atoms fly out of the molecule,” explained Santra. “The rest form fragments of several atoms.” The entire process occurs in about 600 fs. This is still incredibly short with respect to human standards, yet exceptionally long for structural analysis using X-ray lasers.
In the typically 20 femtoseconds of an X-ray laser flash, the atoms move a maximum of 0.1 nanometers—that is in the range of individual atom diameters and smaller than the measurement accuracy of structural analysis.
Robin Santra, Lead Scientist, Center for Free-Electron Laser Science (CFEL), DESY
One nanometer is one-millionth of 1 mm. For analyzing the structure of proteins, scientists often grow small crystals from the biomolecules. Then, the crystal lattice diffracts the bright X-ray laser flash, thus producing a typical diffraction pattern from which the crystal structure as well as the spatial structure of the individual proteins can be calculated.
A protein’s spatial structure unravels details related to its precise function. The protein crystals are highly sensitive and evaporate by means of the X-ray laser flash. Yet, earlier analyses had indicated that the crystal stays intact sufficiently long to produce the diffraction image before evaporation, thereby revealing its spatial structure.
Now, the new research has confirmed that this holds true even for individual molecules that are not bound in a crystal lattice. “Our findings with buckyballs are likely to play a role in most other molecules,” reiterated Santra.
As it is much more difficult to crystallize a number of biomolecules, researchers believe it could be feasible to use X-ray lasers to identify the structure of ensembles of non-crystallized proteins or even individual biomolecules in the future. According to the team, the study outcomes form the basis for an in-depth understanding and quantitative modeling of the radiation damage in biomolecules caused by X-ray laser flashes.
The research involved scientists from the University of Connecticut, Imperial College London, SLAC National Accelerator Laboratory in the United States, University of Gothenburg, University of Texas, French Synchrotron Soleil, Kansas State University, Max Planck Institute for Nuclear Physics, Tohoku University in Japan, University of Potsdam, Max Born Institute, Universität Hamburg, and DESY. CFEL is a joint institution of DESY, the Max Planck Society, and the Universität Hamburg.