May 17 2009
Researchers at the University of Gothenburg, Sweden, have developed a new method to study single cells while exposing them to controlled environmental changes. The unique method, where a set of laser tweezers move the cell around in a microscopic channel system, allows the researchers to study how single cells react to stress induced by a constantly changing environment.
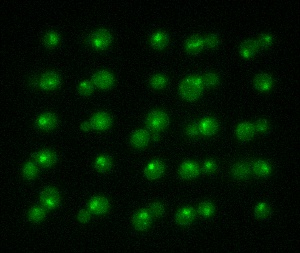 The picture shows yeast cells tagged with a green fluorescent protein that has been stressed with sodium chloride. Photo by Emma Eriksson & Elzbieta Petelenz.
The picture shows yeast cells tagged with a green fluorescent protein that has been stressed with sodium chloride. Photo by Emma Eriksson & Elzbieta Petelenz.
Studies on how cells react to changes in their environment, such as reduced availability of nutrients, have traditionally used cultures consisting of millions of cells. While such studies show how cells on average react to a new environment, they say nothing about individual variation, for example how quickly a single cell responds.
Catches and moves cells
PhD Emma Eriksson and her colleagues at the Department of Physics, University of Gothenburg, Sweden, developed a method where laser tweezers are used to catch a cell the size of about one micrometer, or 0.001 of a mm, and then move the cell between different environments. Placing the cell in a system of channels made of silicone, in which each channel is finer than a human hair, enables the researchers to add and remove substances so that the environment surrounding a single cell changes in a split second - while at the same time watching the reactions through a microscope.
New information
The channels in the so-called microfluidic system can be likened to tiny water pipes. In a channel, a single cell can be exposed to tests and various substances for very exact time periods, which enables the researchers to repeatedly add and remove a substance to see how it affects the behaviour of the cell. This new method gives researchers information that would not be possible to obtain with traditional methods.
How cells survive
In its first stage, the new method has been tested on yeast cells. One of the cells' proteins was tagged with a green fluorescent protein (GFP), enabling researchers to trace the movements of the protein within the cell while it adjusts to a new environment.
'The method can be used to reveal how a cell reacts to stress induced by a change in its environment. The information gained from this may eventually lead to a better understanding of how cells work and what they do to stay alive and healthy in a constantly changing environment', says Eriksson.
The thesis Towards quantitative single cell analysis using optical tweezers and microfluidics was successfully defended at a disputation on April 29th.