Feb 11 2009
Plants, algae, and cyanobacteria (blue-green algae) are masters of everything to do with solar energy because they are able to almost completely transform captured sunlight into chemical energy. This is in part because the electrons set free by the photons are transported out of the "light receptor" 1:1 to be used as the driving force for chemical reactions. Japanese researchers have now developed a new process to capture light energy with nearly equal efficiency. As they report in the journal Angewandte Chemie, they "plug" a molecular "wire" directly into a biological photosynthetic system to efficiently conduct the free electrons to a gold electrode.
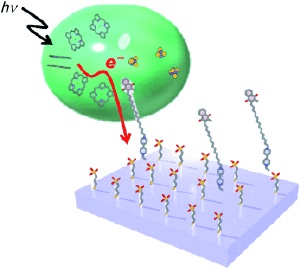
The efficiency of photovoltaic energy conversion is of critical significance for the practical application of solar installations. Theoretically, every photon absorbed should release one electron. Whereas modern solar cells are far from achieving high efficiency, natural photosynthetic systems achieve nearly 100 % quantum yield. To improve the efficiency of synthetic systems, experiments were attempted in which biological light-capturing units were deposited onto electrodes as thin films. However, the transfer of electrons from the light-capturing layer into the circuit in this type of system is so inefficient that most of the electrons don't even make it to the target electrode.
The secret to the success of natural photosystems is the perfect fit of the individual components. The molecules fit precisely together like plugs and sockets and can pass electrons on directly and nearly without loss. The new approach taken by the Japanese researchers cleverly connects photosystem I (PSI) from the blue-green algae Thermosynechococcus elongatus with a synthetic apparatus. An important component of the electron transmission sequence of PSI is vitamin K1. The researchers removed the vitamin K1 from the PSI protein complex and replaced it with a synthetic analogue. This consists of three parts: 1) The same molecular "plug" with which vitamin K1 is bound to the protein complex (napthoquinone group) is used to "plug in" the synthetic binding component to PSI; 2) a molecular "wire" (hydrocarbon chain) with the same length as in vitamin K1 ensures that the binding component protrudes from the protein complex; and 3) at the other end of the wire is an additional "plug" (viologen group) that anchors the ensemble to a specially coated gold electrode. Electrons released by irradiation of PSI and transmitted along the wire are very efficiently transmitted to the gold electrode by the viologen group.
It may be possible to use this new strategy to integrate other biocomponents into synthetic systems.