There is relatively little hard tissue in the human body. Other than our bones and teeth, the rest of our bodies are made of different types of soft tissue, including muscular tissue, lymph vessels, muscles, and nerves. Many diseases affect different regions of the soft tissue, and therefore being able to image these structures is a key part of disease diagnosis.
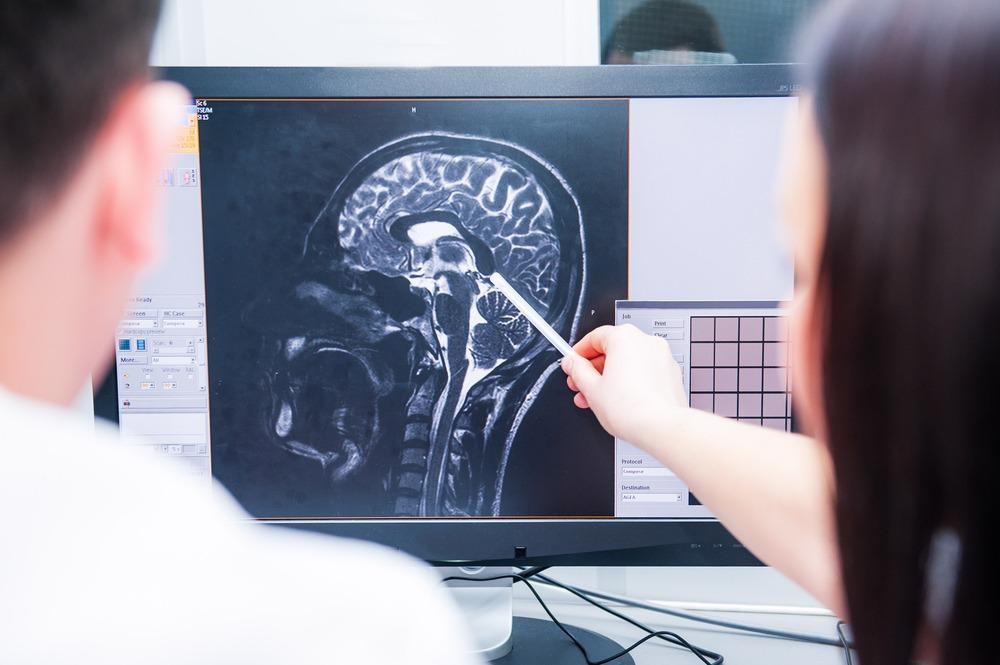
Image Credit: Okrasiuk/Shutterstock.com
Imaging soft tissues, particularly deep body soft tissue, can be very challenging. Imaging hard-tissue structures with good image contrast is somewhat more straightforward as the large difference in density means a significant variation in X-ray attenuation between regions of hard tissue and the surrounding soft tissue. The latter is largely transparent to higher energy X-rays.
Higher energy electromagnetic radiation has a much greater penetration depth than low-energy radiation, which is better absorbed by soft tissues.1 There is a compromise for deep body soft tissue imaging between achieving reasonable signal levels to recover and image while the target radiation can still reach the site of interest.
Optical and Infrared Approaches
An ideal clinical imaging method needs to be non-invasive, rapid and provide results that offer an accurate diagnosis. There is increased interest in more ‘point-of-care’ imaging approaches as these reduce the demand for costly infrastructure and the wait times associated with the laboratory processing of samples.2
Optical imaging methods are some of the most straightforward when designing compact, handheld imaging devices, and are widely used for the diagnosis of tumors and therapeutic monitoring.3
The challenge for deep tissue imaging with optical methods is the ‘biological window’ – this is the wavelength range where the light remains sufficient and unattenuated for deep-tissue imaging and the first of these is between ~700 – 950 nm.4 This is because hemoglobin and other compounds in the body, including water, have very strong absorptions outside of this region.
Attempting to exploit these biological windows and other windows further into the infrared region has led to the development of new imaging systems and fluorophores that operate at longer wavelengths. Key challenges to be overcome also include fluorescence problems and scattering but recent development in image analysis, processing, and imaging geometries are now making it possible to achieve near-cellular spatial resolutions with near-infrared methods for deep-tissue imaging.5
For soft tissue injuries, infrared thermal imaging is also being explored as a way of rapid diagnosis6 and is suitable for deep-tissue injuries.7 The rapid and cost-effective information from this technique may make it an attractive option for assessing diseases characterized by inflammation and infection.
While optical approaches may be limited for in-situ deep-tissue imaging, they still form a key part of laboratory-based histopathological approaches as many diseases, such as cancers, can be diagnosed using optical microscopy to examine changes in the cell structures.8
MRI
For soft tissue imaging and disease diagnosis, particularly when whole-body exploratory scans are required, magnetic resonance imaging (MRI) is often the technique of choice.
MRI has an excellent penetration depth alongside a good spatial resolution, and the high level of information recovered on different soft tissue structures from an MRI scan mean it can be used for diagnosis and to provide information for a prognosis.9 Unlike X-ray methods such as computerized tomography, an MRI scan does not expose a patient to any ionizing electromagnetic radiation.
However, the key drawback of MRI is that it requires very expensive hardware, and the scan times range from minutes to hours for more detailed scans. This is particularly problematic as any motion from the patient can disrupt the quality of the imaging process.
Ultrasound
Ultrasound is another non-invasive technique used to image soft tissue such as nerves.10 Different probe frequencies can be used to vary the penetration depth of the ultrasound to optimize spatial resolution for the target of interest.
Ultrasound is commonly used in the diagnosis of skin diseases and soft tissue infections and can help improve patient outcomes by identifying the presence of fluid at infection sites.11 However, certain structures can be difficult to distinguish, such as the difference between abscesses and hematomas, and rely heavily on the skill of the operator, making a comprehensive diagnosis from an ultrasound alone challenging. This is where the lack of chemical level information can be very problematic for early diagnosis.
Early Disease Diagnosis
Deep-tissue imaging still faces many challenges and often early disease diagnosis relies on a compromise between the expense, difficulty, and accessibility of the technique used. For example, ultrasound imaging can provide indications of potentially serious conditions such as necrotizing fasciitis11. This information, while not a comprehensive diagnosis, can still be used to inform and improve clinical pathways.
Near-infrared imaging is undergoing many rapid developments and is helping to recover chemical-level information on biomarkers present in tumors. This often helps form a very accurate diagnosis.
References and Further Reading
- Fass, L. (2008). Imaging and cancer: A review. Molecular Oncology, 2, 115–152. https://doi.org/10.1016/j.molonc.2008.04.001
- Yager, P., Domingo, G. J., & Gerdes, J. (2008). Point-of-Care Diagnostics for Global Health. Ann. Rev. Biomed. Eng, 10, 107–144. https://doi.org/10.1146/annurev.bioeng.10.061807.160524
- Solomon, M., Berezin, Y., & Achilefu, S. (2011). Optical Imaging in Cancer Research: Basic Principles, Tumor Detection, and Therapeutic Monitoring. Medical Principles and Practice, 20, 397–415. https://doi.org/10.1159/000327655
- Hemmer, E., Benayas, A., Legare, F., & Vetrone, F. (2016). Exploiting the biological windows: current perspectives on fluorescent bioprobes emitting above 1000 nm. Nanoscale Horizons, 1, 168. https://doi.org/10.1039/c5nh00073d
- Dang, X., Bardhan, N. M., Qi, J., Gu, L., Eze, N. A., Lin, C., Kataria, S., Hammond, P. T., & Belcher, A. M. (2019). Deep-tissue optical imaging of near cellular-sized features. Scientific Reports, 9, 3873. https://doi.org/10.1038/s41598-019-39502-w
- Ioannou, S. (2020). Functional Infrared Thermal Imaging: A Contemporary Tool in Soft Tissue Screening. Scientific Reports, 1–9. https://doi.org/10.1038/s41598-020-66397-9
- Ioannou, S. (2020). Functional Infrared Thermal Imaging: A Contemporary Tool in Soft Tissue Screening. Scientific Reports, 10, 9303. https://doi.org/10.1038/s41598-020-66397-9
- Koerner, S., Adams, D., Harper, S. L., Black, J. M., & Langemo, D. K. (2019). Use of Thermal Imaging to Identify Deep-Tissue Pressure Injury on Admission Reduces Clinical and Financial Burdens of Hospital-Acquired Pressure Injuries. Advances in Skin and Wound Care, 32(7), 312–320. https://dx.doi.org/10.1097%2F01.ASW.0000559613.83195.f9
- Scalas, G., Parmeggiani, A., Martella, C., Tuzzato, G., Bianchi, G., Facchini, G., Clinca, R., & Spinnato, P. (2021). Magnetic resonance imaging of soft tissue sarcoma: features related to prognosis. European Journal of Orthopaedic Surgery & Traumatology, 31(8), 1567–1575. https://doi.org/10.1007/s00590-021-03003-2
- Upadhyaya, V., & Choudur, H. N. (2021). Imaging in peripheral neuropathy: Ultrasound and MRI. Indian Journal of Musculoskeletal Radiology, 3(1), 14–23. https://doi.org/10.25259/IJMSR
- Rourke, K. O., Kibbee, N., & Stubbs, A. (2015). Ultrasound for the Evaluation of Skin and Soft Tissue Infections. Emergency Medicine, 112, 3. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc6170135/
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.