Sep 26 2014
Valeria Conti Nibali and Prof Dr Martina Havenith-Newen (Cluster of Excellence RESOLV – Ruhr explores Solvation) made this discovery by using a combination of terahertz absorption spectroscopy and molecular dynamics simulations. The researchers report their findings in the Journal of the American Chemical Society (JACS).
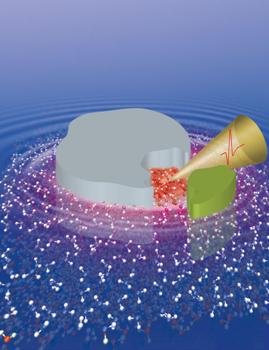 This is a schematic diagram of the hydration funnel in an enzyme-substrate complex (the protein is depicted in grey, its binding partner in green, and the funnel in yellow). Credit: Havenith/Conti Nibali
This is a schematic diagram of the hydration funnel in an enzyme-substrate complex (the protein is depicted in grey, its binding partner in green, and the funnel in yellow). Credit: Havenith/Conti Nibali
Choreography of water movements
New experimental technologies, such as terahertz absorption spectroscopy, pave the way for studies of the dynamics of water molecules surrounding biomolecules. Using this method, the researchers proved some time ago that proteins influence water molecules in their surroundings: they determine the choreography of their movements. This effect occurs not only in the immediate vicinity of the protein, but can also be detected in the remote layers of the surrounding water molecules.
Collective interaction helps choose binding partner
But what purpose would such an interaction have? The researchers have come closer to finding an answer to this question by employing molecular dynamics simulations. It was demonstrated that the movement of water molecules in the vicinity of the protein's active centre, the so-called binding pocket, is connected to potential binding partners in the water solvent. "This movement causes the water molecules to form a hydration funnel of sorts, making up part of the molecular recognition mechanism in both partners," explains Prof Dr Martina Havenith-Newen. Moreover, the movements of the water molecules have proved to be specific for certain binding partners. Thus, if there are different potential binding candidates in the solvent, all competing to bind to the protein, these collective water movements are thought to assist binding. To conclude, such correlated water movements could support the interaction of biomolecules like enzymes and proteins with their binding partners and play a significant role in their mutual recognition, allowing the biomolecule to select or reject certain binding partners.