May 10 2019
Photocathodes and solar cells composed of copper oxide (Cu2O) might theoretically attain high efficiencies for solar energy conversion. In reality, however, large losses happen. At present, a team of researchers at the HZB have been able to use a high-tech femtosecond laser experiment to establish where these losses occur: not so much at the interfaces, but rather far more in the interior of the crystalline material. These results offer clues on how to enhance Cu2O and other metal oxides for applications as energy materials.
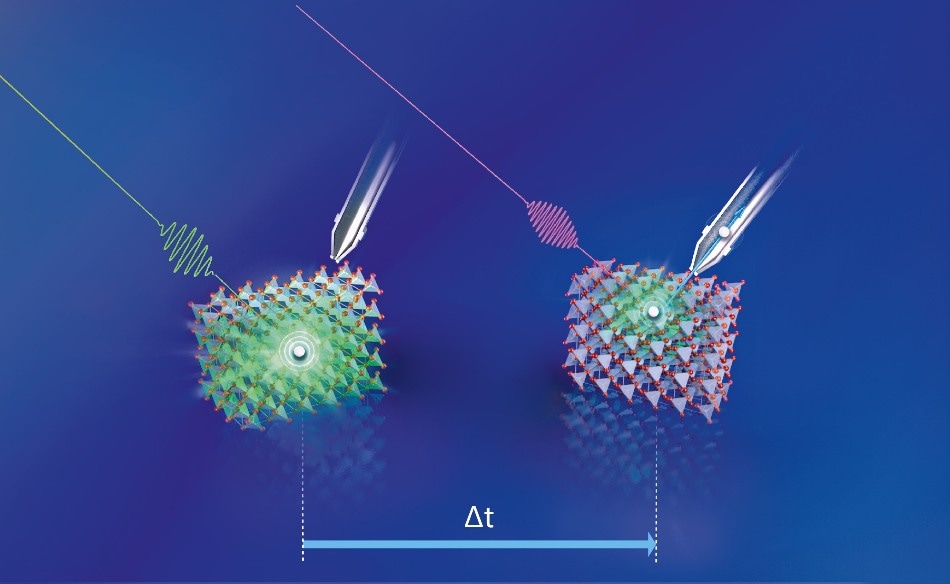 A green laser pulse initially excites the electrons in the Cu2O; just fractions of a second later, a second laser pulse (UV light) probes the energy of the excited electron. (Copyright: M. Künsting/HZB)
A green laser pulse initially excites the electrons in the Cu2O; just fractions of a second later, a second laser pulse (UV light) probes the energy of the excited electron. (Copyright: M. Künsting/HZB)
Cu2O is a very favorable option for future solar energy conversion: as a photocathode, the Cu2O (a semiconductor) might be able to utilize sunlight to electrolytically split water and therefore, produce hydrogen, a fuel that can chemically store the solar energy.
Loss processes at the interface?
Cu2O consists of a band gap of 2 electron volts, which ties up very well with the energy spectrum of sunlight. Faultless copper oxide crystals should hypothetically be able to offer a voltage close to 1.5 volts when illuminated with light. The material would, therefore, be ideal as the top-most absorber in a photoelectrochemical tandem cell for water splitting. A solar-to-hydrogen energy conversion efficiency of up to 18% should be attainable. However, the real values for the photovoltage lie significantly below that value, inadequate to create copper oxide an efficient photocathode in a tandem cell for water splitting. Thus far, loss processes close to the surface or at boundary layers have been largely held accountable for this.
Experiments in the femtosecond laser lab
Researchers at the HZB Institute for Solar Fuels have currently studied these processes closely. The team received superior-quality Cu2O single crystals from colleagues at the well-known California Institute of Technology (Caltech), then vapor-deposited a very thin, transparent layer of platinum on them. This platinum layer serves as a catalyst and boosts the efficiency of water splitting. They analyzed these samples in the femtosecond laser laboratory (1 fs = 10-15 s) at the HZB to learn what processes cause the loss of charge carriers and especially whether these losses take place in the interior of the single crystals or at the interface with the platinum.
Defect states detected
A green laser pulse originally excited the electrons in the Cu2O; merely fractions of a second later, a second laser pulse (UV light) measured the energy of the excited electron. The researchers were then able to recognize the core mechanism of photovoltage losses via this time-resolved two-photon photon emission spectroscopy (tr-2PPE). “We observed that the excited electrons were very quickly bound in defect states that exist in large numbers in the band gap itself”, reports first author Mario Borgwardt, who is currently continuing his work as a Humboldt fellow at Lawrence Berkeley National Laboratory in the USA. The study’s coordinator, Dennis Friedrich, explains: “This happens on a time scale of less than one picosecond (1 ps = 10-12 s), i.e. extremely fast, especially compared to the time interval charge carriers need to diffuse from the interior of the crystalline material to the surface.”
Loss processes mainly in the bulk
“We have very powerful experimental methods at the femtosecond laser laboratory of the HZB for analysing energy and dynamics of photo-excited electrons in semiconductors. We were able to show for copper oxide that the losses hardly occur at the interfaces with platinum, but instead in the crystal itself”, says Rainer Eichberger, initiator of the study and head of femtosecond spectroscopy lab.
Excellence Cluster UniSysCat
These new insights are our first contribution to the UniSysCat Excellence Cluster at the Technische Universität Berlin, in which we are a partner.
Roel van de Krol, Head of Institute for Solar Fuels, HZB
UniSysCat concentrates on catalytic processes that occur over very varied time scales: while charge carriers react very rapidly to excitations by light (femtoseconds to picoseconds), chemical processes such as (electro)catalysis require many orders of magnitude more time (milliseconds). An efficient photochemical conversion necessitates that both processes be enhanced together. The present results that have recently been published in the distinguished journal Nature Communications are a crucial step in this direction.