Researchers at the University of Regensburg have discovered a technique for controlling the quantum state of individual electrons by employing a high-resolution atomic microscope.
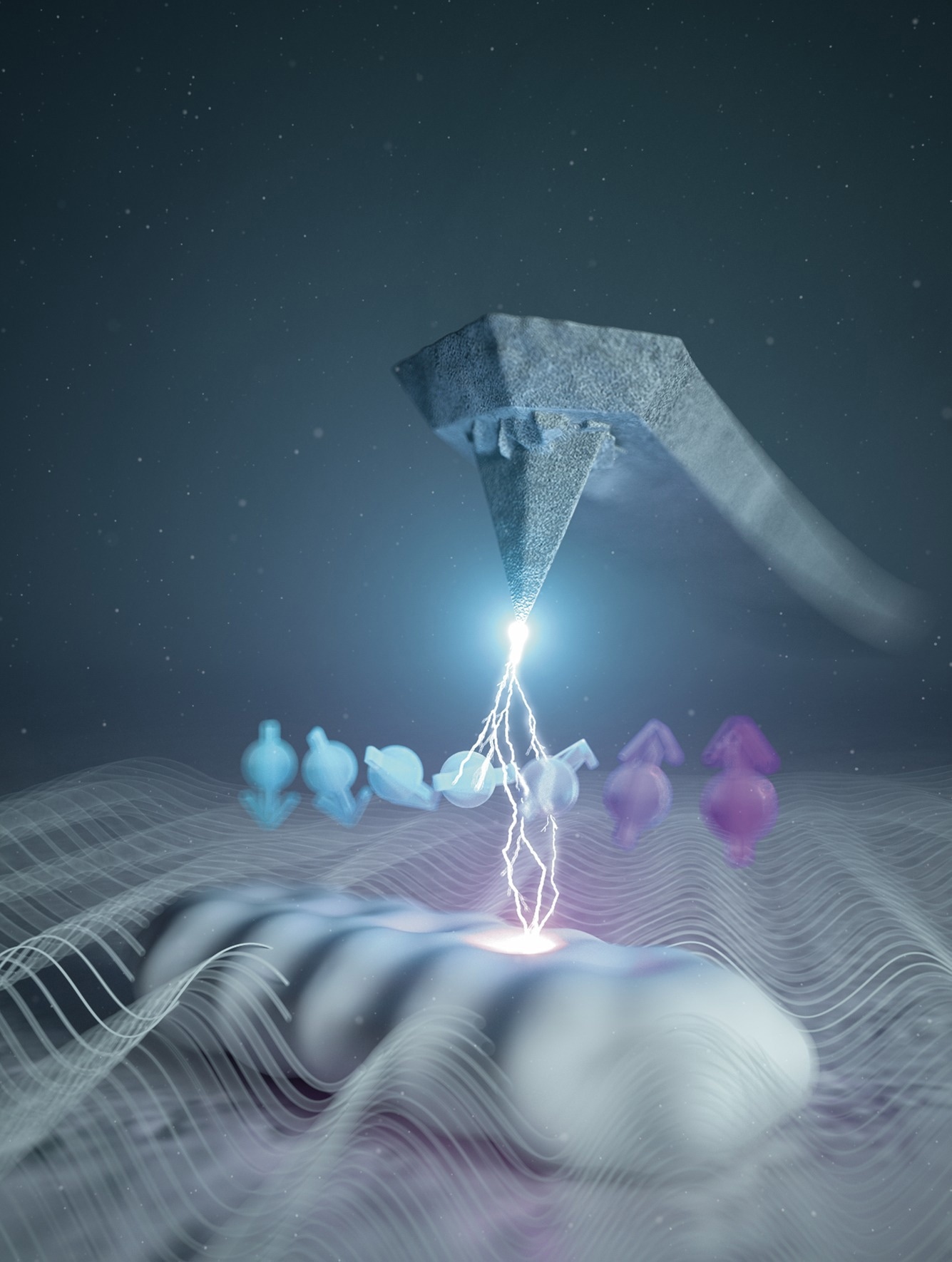 Artistic illustration of the integration of electron spin resonance in atomic force microscopy. The white structure at the bottom represents a single molecule, the arrows its spin quantum state, and the wavy lines the radio-frequency magnetic field needed for the electron spin resonance, which is detected by the tip of the atomic force microscope. Image Credit: Eugenio Vázquez.
Artistic illustration of the integration of electron spin resonance in atomic force microscopy. The white structure at the bottom represents a single molecule, the arrows its spin quantum state, and the wavy lines the radio-frequency magnetic field needed for the electron spin resonance, which is detected by the tip of the atomic force microscope. Image Credit: Eugenio Vázquez.
The findings of the research have been officially published in the journal Nature. Everything in the surroundings, including ourselves, is comprised of molecules. These molecules are so minuscule that even a tiny speck of dust contains an immense number of them.
What makes this even more intriguing is the fact that modern technology allows one to accurately visualize such molecules using an atomic force microscope. Unlike an optical microscope, this instrument operates by sensing minute forces between a tip and the molecule under examination.
This technique enables the imaging of a molecule's internal structure. However, observing a molecule in this manner does not provide a comprehensive understanding of all its distinct properties. For example, determining the types of atoms composing the molecule is already a challenging task.
Fortunately, there are alternative tools available for deciphering the composition of molecules. One such method is electron spin resonance, which shares similarities with the principles employed in medical MRI scanners.
In electron spin resonance, a large number of molecules are typically required to generate a detectable signal. Consequently, this approach only yields information about the average properties of the molecules, not each individual one.
Researchers at the University of Regensburg, led by Prof. Dr. Jascha Repp from the Institute of Experimental and Applied Physics at the UR, have taken a significant step by integrating electron spin resonance into atomic force microscopy.
Notably, the electron spin resonance is directly detected with the microscope's tip, generating a signal from a single, individual molecule. This breakthrough enables the characterization of single molecules on a one-by-one basis, facilitating a straightforward determination of the atoms composing the imaged molecule.
We could even discriminate molecules that do not differ in the type of atoms, that they were composed of, but only in their isotopes, namely, in the composition of the atoms' nuclei.
Lisanne Sellies, Study First Author, University of Regensburg
“Yet, we are even more intrigued by another possibility that electron spin resonance entails: this technique can be used to operate the spin-quantum state of the electrons present in the molecule,” explained Professor Dr. Repp.
Quantum computers store and process information encoded in a quantum state. Executing calculations on quantum computers requires manipulating a quantum state without succumbing to information loss due to what is known as decoherence.
The researchers in Regensburg demonstrated that their innovative technique enables the manipulation of the quantum state of the spin in a single molecule multiple times before decoherence occurs.
Given that the microscopy technique allows for the imaging of the individual surroundings of the molecule, this newly developed method could contribute to understanding how decoherence in a quantum computer is influenced by the atomic-scale environment and, ultimately, how to prevent it.
This project received funding from the ERC Synergy Grant MolDAM (no. 951519) and the German Research Foundation (no. RE2669/6-2).
Journal Reference:
Sellies, L., et al. (2023) Single-molecule electron spin resonance by means of atomic force microscopy. Nature. doi.org/10.1038/s41586-023-06754-6.
Source: https://www.uni-regensburg.de/en