Nov 10 2017
Radioactivity plays a vital role in medical research though it may have a bad rap. A groundbreaking new technique to produce radioactive molecules, founded in the lab of Princeton chemistry professor David MacMillan, is capable of bringing new medicines to patients much faster than ever before.
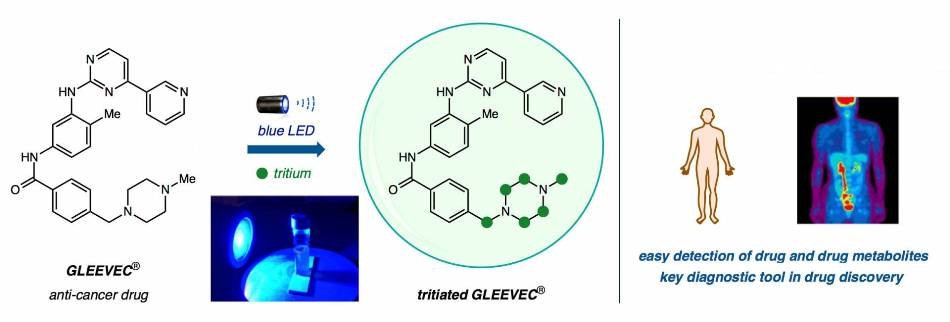 From left: Gleevec, an anti-cancer drug, is submerged in heavy water (T2O) and bathed in blue LED light to replace hydrogen atoms with tritium atoms (green circles) in a one-step direct hydrogen isotope exchange (HIE). Clinicians can trace radioactive compounds in the body using sophisticated imaging technologies for research and diagnostic purposes. (Image courtesy of the researchers)
From left: Gleevec, an anti-cancer drug, is submerged in heavy water (T2O) and bathed in blue LED light to replace hydrogen atoms with tritium atoms (green circles) in a one-step direct hydrogen isotope exchange (HIE). Clinicians can trace radioactive compounds in the body using sophisticated imaging technologies for research and diagnostic purposes. (Image courtesy of the researchers)
Your average drug takes 12 to 14 years to come to market, so everything that we can do to take that 14- or 12-year time frame and compress it is going to advantage society, because it gets medicines to people — to society — so much faster.
David MacMillan, the James S. McDonnell Distinguished University Professor of Chemistry.
Every promising new medication needs to go through testing in order to confirm that it affects the part of the body it has been intended to affect. “Is it going to the right place? The wrong place? The right place and the wrong place?” MacMillan asked.
Locating the path of a chemical that dissolves into the bloodstream presented a grave challenge, but one that radiochemists solved many years ago by exchanging individual atoms with radioactive substitutes. After performing that, “the properties of the molecule — of the drug — are exactly the same except that they’re radioactive, and that means that you can trace them really, really well,” MacMillan said.
However, that presented a fresh problem.
“Getting these radioactive atoms into the drug is not a trivial thing to do,” he said. “People have developed long, sometimes month-long, two-month, three-month long sequences just to get a tiny amount of a substance with a few radioactive atoms.”
He and his colleagues have discovered a better way, drawing on their work employing blue LED lights and catalysts capable of responding to light, called photocatalysts. Their research has been published online in the Nov. 9 edition of the journal Science.
“It was a wacky idea! Fortunately, it worked,” MacMillan said. “What we came up with was, if you shine light on them, and you have a photocatalyst, could these photocatalysts actually remove the non-radioactive atom and then install the radioactive atom?”
They could.
MacMillan’s technique makes use of “heavy water,” capable of replacing the hydrogen (H) in H2O with tritium, a radioactive version of hydrogen that has an additional two neutrons per atom.
If you just let your drug sit in the radioactive water and shine light on it with a catalyst, the catalyst will remove the atom which is not radioactive — in this case it’s hydrogen — and replace it with tritium,
David MacMillan, the James S. McDonnell Distinguished University Professor of Chemistry.
Suddenly, joining one of these atomic labels takes hours and not months, and the technique works on several kinds of commonly used compounds. The researchers have previously tested it on 18 commercially available medicines, and also on candidates in the Merck drug discovery pipeline.
For compounds that do not require radioactive tags, the same one-step process can be used for swapping in deuterium, a version of hydrogen with just one extra neutron. These “radio labels” (with tritium) and “stable labels” (with deuterium) have innumerable applications, in academia and also in drug discovery.
The simplicity of this new technique has another implication, stated Jennifer Lafontaine, the senior director of synthesis and analytical chemistry for Pfizer in La Jolla, California, who was not part of the research.
Since the earlier process was so resource intensive, deuterium- or tritium-labeled molecules were frequently only developed for chemicals that were “quite advanced in the drug discovery process,” she said. “This methodology could therefore open the door to earlier and expanded use of isotopic labeling in drug discovery, significantly enhancing our ability to study drug candidates on a deeper level, and across a range of applications.”
This new technique influences the growing field of photocatalysis pioneered at Princeton and applied it to another new field, MacMillan stated. It also has noticeable financial value, but he waved that off.
“No one’s patenting any of this, because we want it to be available for everyone to use,” MacMillan said.
This technology was produced in association with Merck at Princeton’s Merck Catalysis Center, where Princeton graduate student Yong Yao Loh and postdoctoral researcher Kazunori Nagao carried out research using the radioactive material, said Ian Davies, a co-author on the Science paper who was the key investigator at the partner lab at Merck while the research was being executed.
“This is a great example of a Princeton-industrial collaboration that benefits science and all of society,” Davies said.
The research was financially supported by the National Institutes of Health (R01 GM103558-04 to D.W.C.M., Y.Y.L. and K.N.), a graduate fellowship from the Agency for Science, Technology and Research (Y.Y.L.), and the Japan Society for the Promotion of Science for a postdoctoral fellowship (K.N.).