Apr 13 2015
Scientists from Jülich, Grenoble, Frankfurt and Moscow uncovered the atomic structure of KR2, a light-driven transporter for sodium ions which had only recently been discovered. Based on the structural information the team then identified a simple way to turn KR2 from a sodium- into a potassium pump using simple means. Integrated into neurons, this could make KR2 a valuable tool for optogenetics, a new field of research that uses light-sensitive proteins as molecular switches to precisely control the activity of neurons and other electrically excitable cells using light impulses.
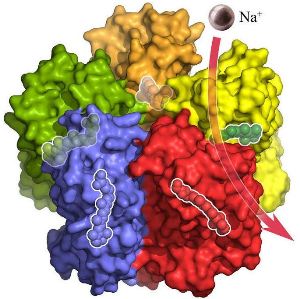 The surface of the KR2 complex shown from the side. Each of the five KR2 molecules binds and transports a sodium ion (purple) across the membrane. © Forschungszentrum Jülich/IBS Grenoble
The surface of the KR2 complex shown from the side. Each of the five KR2 molecules binds and transports a sodium ion (purple) across the membrane. © Forschungszentrum Jülich/IBS Grenoble
In 2013, scientists made an unexpected discovery while investigating the marine bacterium Krokinobacter eikastus. In its cellular membrane, the bacterium had a previously unknown type of ion transporter. The protein, which was dubbed KR2, belongs to a group of light-sensitive proteins that have become the basis of the research field of optogenetics. When exposed to light, these proteins allow charged particles to flow into the cell or transport them outside the cell.
Integrating these ion transporters into the neuronal membrane makes it possible to alter their state of charge using light impulses, thus enabling their activity to be precisely controlled. This method quickly became established in the neurosciences, in particular. However, only a few proteins are currently available for this and each of these proteins was only permeable to certain ions.
KR2 transports positively charged sodium ions out of the cell, which is a feature that so far had been missing in the toolkit of optogenetics. However, until now neither the exact atomic structure nor the ion transport mechanism had been known – which is an important prerequisite for utilizing KR2 and adapting it for specific applications. This challenge awakened the interest of a team of structural biologists headed by Valentin Gordeliy, who heads research groups at the Institute of Complex Systems (ICS-6) at Forschungszentrum Jülich, Germany, at the Institute de Biologie Structurale in Grenoble, France, and at the Moscow Institute of Physics and Technology in Russia. Using X-ray crystallography, the team obtained the first high-resolution 3D structural images of the single protein and the five-part complex that the KR2 molecule spontaneously forms under physiological conditions.
“The structure of KR2 has many unique features,” says Ivan Gushchin, one of the lead authors of the study and a postdoc of Gordeliy. One of these features is a short protein helix capping the outfacing opening of the pump like a lid. A feature of KR2, that the scientists were particularly interested in was the unusual structure of the inward facing ion-uptake cavity, which was found to be unusually large and protruding from the protein surface. “We hypothesized that this structure could act as a kind of filter causing the selectivity of KR2 for sodium ions,” Gushchin explains.
To put this idea to the test, Gordeliy´s team changed the structure by swapping specific amino acids at the site in question through targeted mutations. Not only did KR2 indeed lose its sodium-pumping ability; but also one of the mutations seemed to turn KR2 into a light-driven potassium pump – the first of its kind. To accurately prove this observation the team performed a series of electrophysiological experiments with the purified protein in collaboration with Ernst Bamberg at the Max Planck Institute of Biophysics in Frankfurt am Main, who is an expert on membrane proteins and one of the founders of optogenetics.
For potential optogenetic application, this result is especially interesting, says Bamberg: “In neurons, transporting potassium ions from the cell is the natural mechanism of deactivation. Normally, an activated neuron will release them through passive potassium channels in the membrane.” With a light-activated, active potassium pump this process could be precisely controlled”. This would make KR2 a very effective off-switch for neurons. Now, ways of integrating the pump into different types of cells need to be developed. “In combination with the light-activated Channelrhodopsin 2, which is used in labs worldwide as a molecular off-switch, the KR2 potassium pump would then form a perfect pair of tools for the precise control of nerve cell activity,” says Bamberg.
Source: http://www.mpg.de/