Advances in multiphoton microscopy now allow scientists to follow the activity of multiple neurons as a mouse sees, responds and behaves.
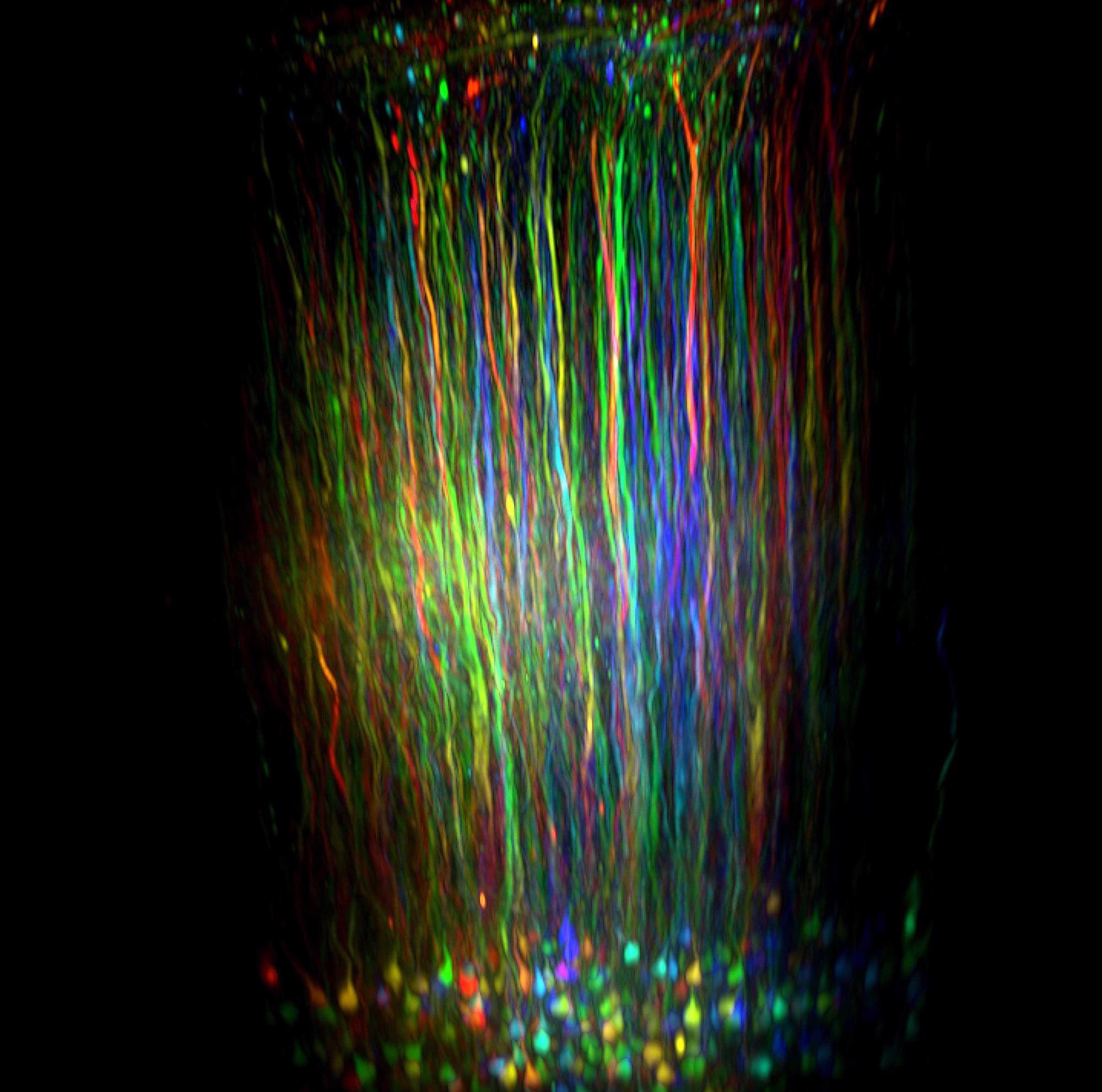
Image Credit: Sutter Instruments
The question of how the firing patterns in specific neural circuits determine an animal's behavior has been at the forefront of neuroscience research for decades.
Initial studies on excised brain slices offered a partial answer, suggesting that firing patterns of individual neurons may alter during activity. Bernd Kuhn's work looks to better answer this question, as he seeks to visualize neural firing patterns in an animal's brain as it performs various behaviors or reacts to stimuli.
In order to do this, Kuhn - a neuroimaging expert at the Okinawa Institute of Science and Technology Graduate University in Japan – was required to expand the capabilities of the multiphoton microscopy imaging method.
Multiphoton microscopy was first developed in the 1990s, and since then it has been used to help reveal cellular changes during neurodegenerative disease or brain development in animal models.
As the technique has improved, it has offered new insight into the function of the cerebral cortex (the brain's outermost layer) which is responsible for a range of essential and higher brain functions.
Kuhn's goal was to help neuroscientists better understand what goes wrong in mouse models of Parkinson's, Alzheimer's and other brain disorders. This was a challenging task, requiring the development of fundamental knowledge on how the brain integrates motor, sensory and cognitive information as mice performed a series of actions.
Gaining this level of insight required the observation of a deep, previously inaccessible layer of the visual cortex that communicates with an even deeper layer of the brain called the thalamus. Observations were performed for over an hour as each mouse slept, woke or, ran.
Kuhn's efforts were successful. These experiments involved a mouse running on a moving ball to reach a reward of water. The mouse's head was fixed in place and a behavioral camera observed its eyes in order to gauge alertness. Measurements were also acquired detailing parameters such as how often the mouse licked its whiskers.
These behaviors were matched with neural activity from over two hours of observations - long enough for the mouse to become tired and fall asleep. Kuhn and his colleagues reported their findings in the Current Biology journal in October 2020.1
Kuhn highlighted that the experiment needed to last that long in order to observe the various behavioral states:
"It's a beginning for us to find a connection between behavior and neuronal activity. You need this as a base for understanding what is going wrong if you have any neuronal disease."
Penetrating Vision
The use of multiphoton microscopy has allowed scientists to address an enduring issue in biological microscopy – the need to add fluorescent markers to cells to help spotlight certain structures. In confocal microscopy - the state-of-the-art method prior to the advent of multiphoton microscopy - the visible light used to excite these fluorophores could cause damage to brain tissue.
In the early 1990s, Winfried Denk and the late Watt Webb at Cornell University discovered a means of mitigating that damage by exciting fluorophores with infrared light rather than visible light.
As infrared light is less energetic, it takes two photons rather than one to excite the fluorophore. However, its less energetic nature means that it can penetrate deeper into the brain without causing damage to tissue.
This innovative advancement allowed neuroscientists to label specific types of neurons with a fluorescent protein or another fluorescent marker, illuminating buried neural circuits by shining infrared light through a transparent window inserted into the animal's skull.
In order to monitor neural circuits while tracking behaviour, Kuhn leveraged the power of an imaging platform he had spent five years developing in partnership with Novato, California-based scientific instrument company, Sutter Instruments.
This new platform incorporates Sutter's movable objective microscope (MOM) - a two-photon instrument that can facilitate spatial scanning by moving an objective through space while utilizing lasers to spotlight one small area at a time.
The platform also records up to three hours' behavior, employing the company's proprietary MScan software to control the experiment and integrate the data.
Using the MScan software it is possible to build up a 3D picture of as many as 10,000 neurons over a wide tissue area. This capability helped Daniel Dombeck, a neuroscientist at North-Western University in Evanston, Illinois, and his team make a startling discovery.
In order to pinpoint circuits that could potentially allow a mouse to monitor its own activity, the researchers placed each mouse on a rolling ball under the microscope. The mouse's head was fixed in place and a virtual-reality maze was projected around it. The researchers recorded the mouse's neural activity as it ran through the virtual maze, determining which brain circuits were in use.
The study showed a circuit that lit up when a mouse was active, as well as revealing nearby neural circuits that lit up once the animal had stopped moving. The team discovered that those nearby circuits kept rough track of how long the mouse had rested. Dombeck and his team reported their findings in the journal Neuron in 2014.2
These newly discovered circuits exist in the part of the brain where Alzheimer's begins. Dombeck suggested that because healthy people can approximate time better than those with early-stage Alzheimer's, observation of these circuits could lead to a straightforward test for the disease's early stages.
Visualizing the Deeper Brain
Researchers are now beginning to excite buried fluorophores with three photons that have even longer wavelengths and lower energy in order to explore even deeper layers of the brain.
Two-photon microscopy employs 800-1100 nm photons that are able to penetrate 0.5-1.0 mm into the brain. Chris Xu, a physicist at Cornell University, and his colleagues have successfully employed three photons with wavelengths of 1700 nm. These wavelengths are able to penetrate up to 1.5 mm into brain tissue without scattering.
This method also focuses the excitation light more sharply, meaning that there is less stray light that could blur the image.
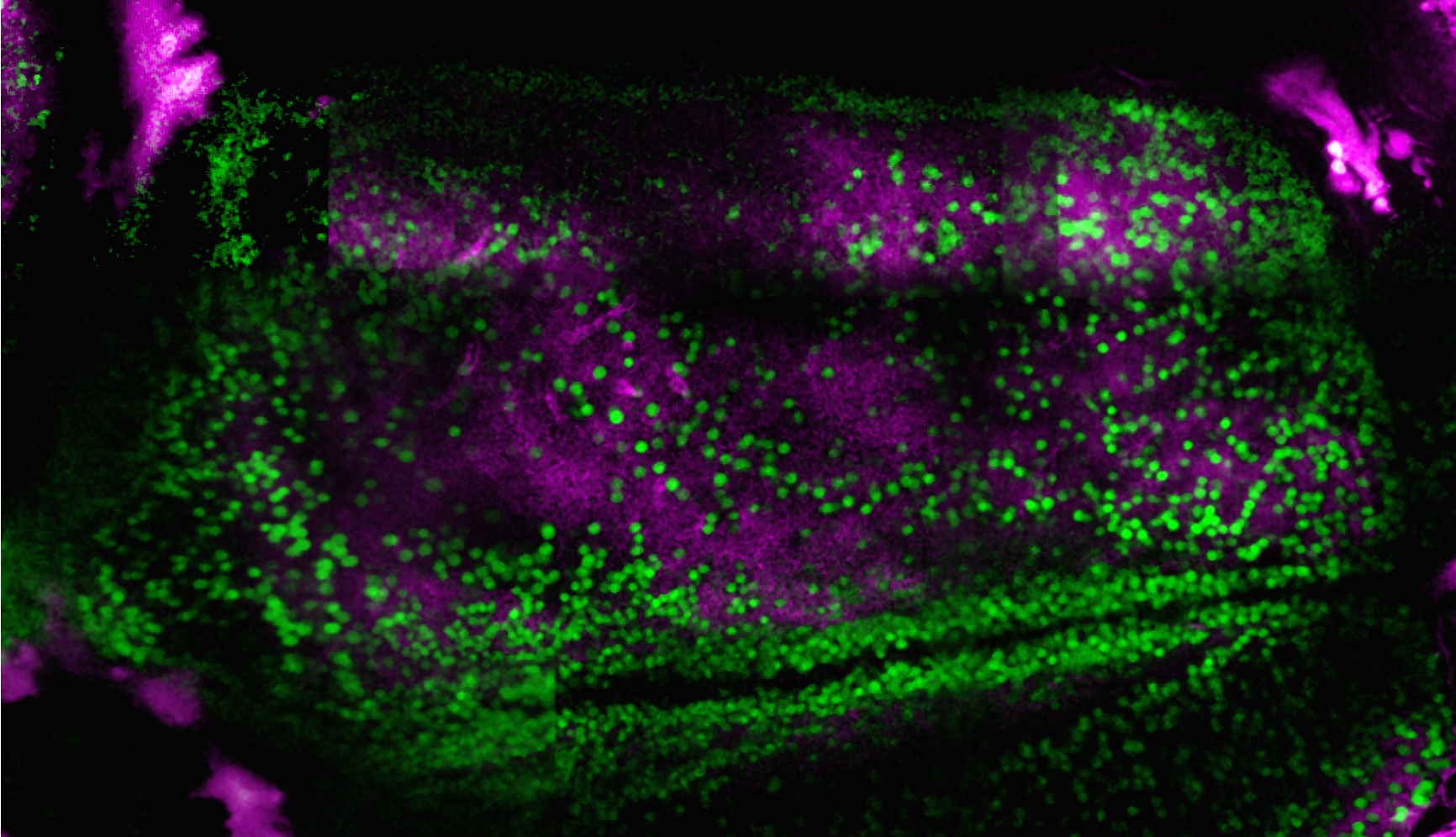
Image Credit: Sutter Instruments
That additional depth is actually quite important. We're able to access much of cortex with a two-photon microscope. But certainly, there are circumstances where you really can't get to the deeper layers of that network. Then you may need to go to the three-photon.
Jack Waters, A Researcher, Allen Institute for Brain Science.
Three-photon microscopy is currently reliant on custom-built or heavily customized setups. It is able to see through a skull and image approximately 0.5 mm into the brain, however. Xu and neurobiologist Joseph Fetcho – a researcher who studies autism - recently utilized a three-photon microscope to image the brain of a live adult zebrafish.3
Zebrafish are popular subjects in studies into brain development because their skulls are translucent when young, becoming opaque as the zebrafish ages. Looking through their skulls could allow researchers to compare an adult with a young zebrafish.
Three-photon microscopy can also be used to investigate the brains of larger animals. Waters' colleagues have been aiming two-photon microscopes at a macaque's visual cortex, which shares some similarities with that of a human.
This method only penetrates up to layer two of the six-layer cortex, however, and most of the activity of interest occurs in layers four and five. Regarding the three-photon technique, Waters says, "we’re expecting to be able to image most of the way, perhaps all the way, through the cortex.”.
Lasers utilized in multiphoton microscopy, the fluorescent labels and the methods of delivering them continue to advance and evolve. This technology can now be used to visualize neurons and synapses across larger brain volumes in multiple areas of the brain.
As the technology continues to improve and the industry offers increasing numbers of turnkey systems, more questions in brain science will be answered.
There are many people doing this. It’s just too exciting for only a few people to do this.
Kuhn, Neuroimaging Expert, Okinawa Institute of Science and Technology Graduate University in Japan
References
- Augustinaite, S., Kuhn, B. Complementary Ca2+ activity of sensory activated and suppressed layer 6 corticothalamic neurons reflects behavioral state. Current Biology 30, 1–16 (2020).
- Heys, J.G., Rangarajan. K.V., Dombeck, D.A. The functional micro-organization of grid cells revealed by cellular-resolution imaging. Neuron 84, 1079–1090 (2014).
- Chow, D.M. et al. Deep three-photon imaging of the brain in intact adult zebrafish. Nature Methods 17, 605–608 (2020).

This information has been sourced, reviewed and adapted from materials provided by Sutter Instruments .
For more information on this source, please visit Sutter Instruments .